
Facile Synthesis of Chiral Arylamines, Alkylamines and Amides by Enantioselective NiH‐Catalyzed Hydroamination - Meng - 2021 - Angewandte Chemie - Wiley Online Library
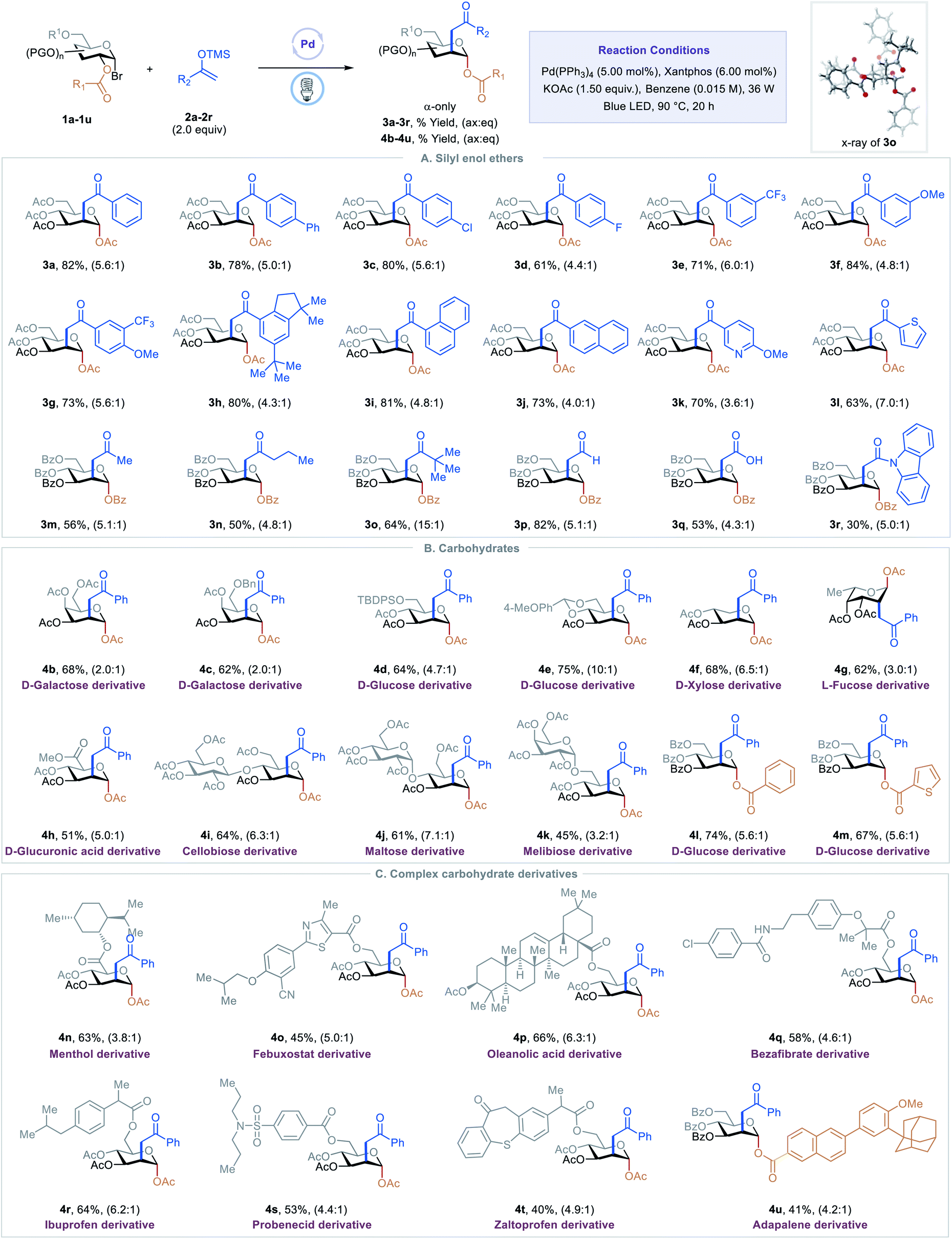
C2-ketonylation of carbohydrates via excited-state palladium-catalyzed 1,2-spin-center shift - Chemical Science (RSC Publishing) DOI:10.1039/D2SC01042A
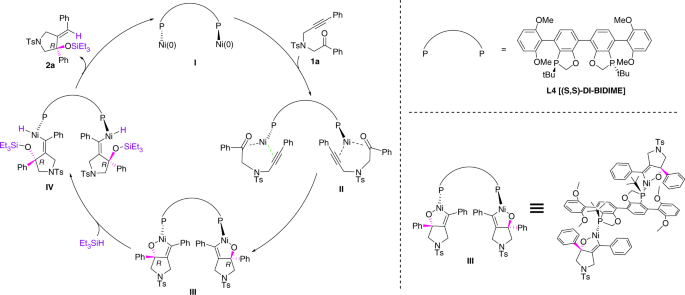
Pyrrolidines and piperidines bearing chiral tertiary alcohols by nickel-catalyzed enantioselective reductive cyclization of N-alkynones | Communications Chemistry
18 Supramolecular coordination chemistry - Annual Reports Section "A" (Inorganic Chemistry) (RSC Publishing) DOI:10.1039/B102906C
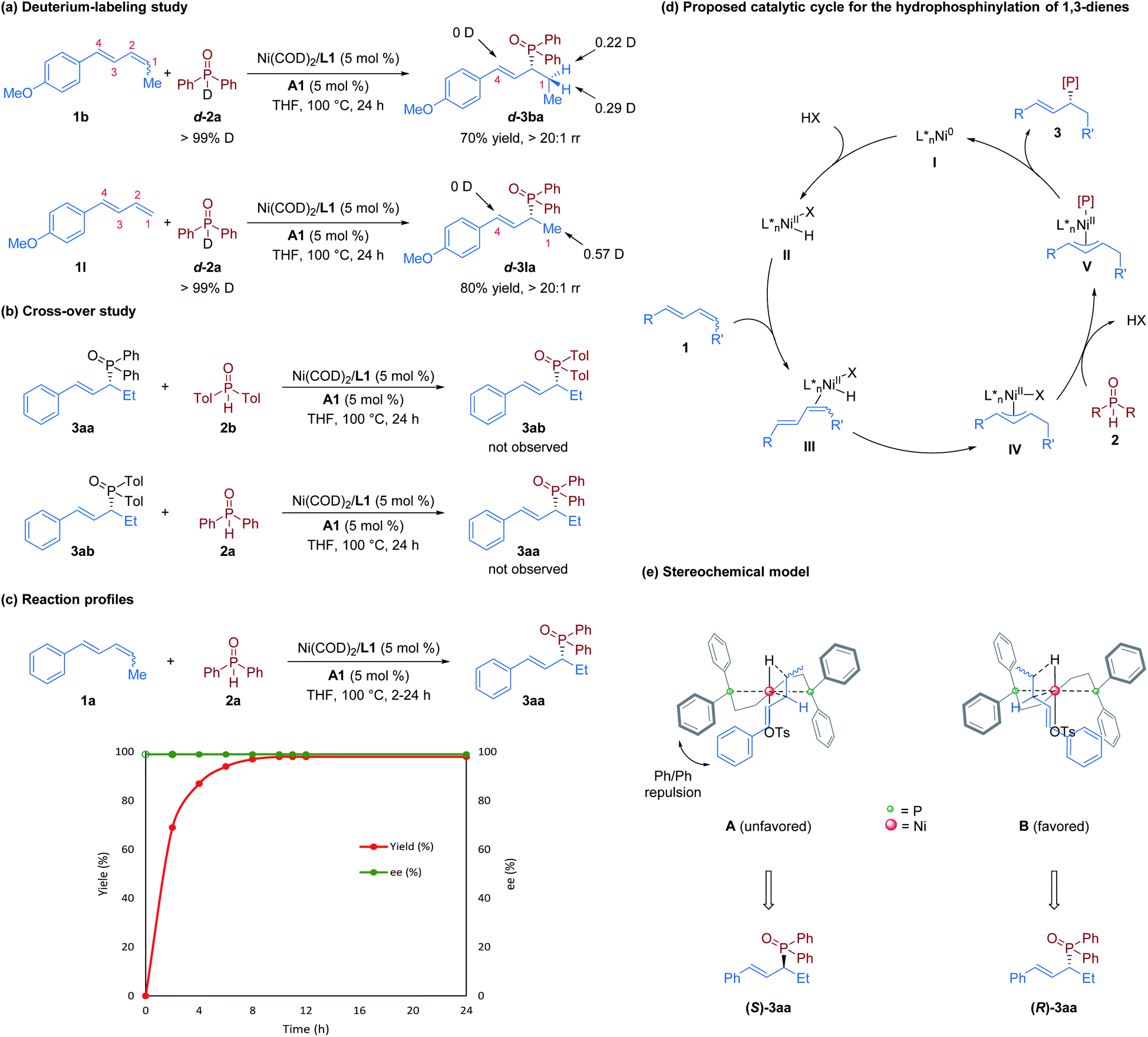
Nickel/Brønsted acid dual-catalyzed regio- and enantioselective hydrophosphinylation of 1,3-dienes: access to chiral allylic phosphine oxides - Chemical Science (RSC Publishing) DOI:10.1039/D1SC05651D

Facile Synthesis of Chiral Arylamines, Alkylamines and Amides by Enantioselective NiH‐Catalyzed Hydroamination - Meng - 2021 - Angewandte Chemie International Edition - Wiley Online Library

Transition-Metal (Pd, Ni, Mn)-Catalyzed C–C Bond Constructions Involving Unactivated Alkyl Halides and Fundamental Synthetic Building Blocks | Accounts of Chemical Research

Nickel-catalysed migratory hydroalkynylation and enantioselective hydroalkynylation of olefins with bromoalkynes. - Abstract - Europe PMC

Facile Synthesis of Chiral Arylamines, Alkylamines and Amides by Enantioselective NiH‐Catalyzed Hydroamination - Meng - 2021 - Angewandte Chemie - Wiley Online Library

Development and Mechanistic Studies of (E)-Selective Isomerization/Tandem Hydroarylation Reactions of Alkenes with a Nickel(0)/Phosphine Catalyst | ACS Catalysis
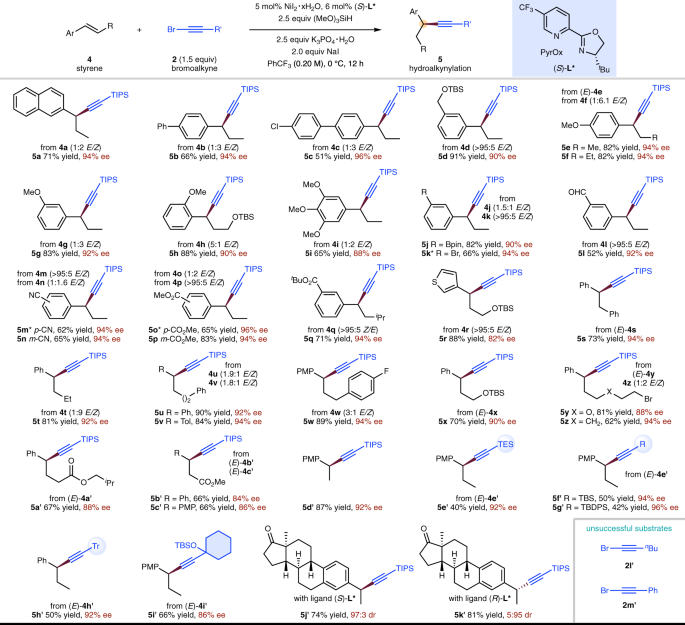
Nickel-catalysed migratory hydroalkynylation and enantioselective hydroalkynylation of olefins with bromoalkynes | Nature Communications

Covalent Modifiers: A Chemical Perspective on the Reactivity of α,β-Unsaturated Carbonyls with Thiols via Hetero-Michael Addition Reactions. - Abstract - Europe PMC

Regio- and enantioselective remote hydroarylation using a ligand-relay strategy | Nature Communications

Iron‐Electrocatalyzed C−H Arylations: Mechanistic Insights into Oxidation‐Induced Reductive Elimination for Ferraelectrocatalysis - Zhu - 2019 - Chemistry – A European Journal - Wiley Online Library

Low-Valent, High-Spin Chromium-Catalyzed Cleavage of Aromatic Carbon–Nitrogen Bonds at Room Temperature: A Combined Experimental and Theoretical Study | Journal of the American Chemical Society
18 Supramolecular coordination chemistry - Annual Reports Section "A" (Inorganic Chemistry) (RSC Publishing) DOI:10.1039/B102906C

A biomimetic SH2 cross-coupling mechanism for quaternary sp3-carbon formation. - Abstract - Europe PMC

Copper-Catalyzed Enantioselective Propargylic Amination of Propargylic Esters with Amines: Copper−Allenylidene Complexes as Key Intermediates | Journal of the American Chemical Society

Visible Light Photocatalysis: Applications and New Disconnections in the Synthesis of Pharmaceutical Agents | Organic Process Research & Development








